Adenosine 5-Monophosphate Activated Protein Kinase (AMPK) Modulators Therapeutics - Pipeline Analysis 2018
Pramod KmrSeptember 27, 2019Adenosine 5-Monophosphate Activated Protein Kinase (AMPK) Modulators Therapeutics - Pipeline Analysis 2018, and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents
1 comment

Adenosine
5-monophosphate activated protein kinase (AMPK) plays an important role
in regulation of homeostasis. Dysregulation of AMPK can result in many diseases
which includes obesity and type 2 diabetes. Several studies have also suggested
that AMPK are also associated with the development of neurological diseases and
cancer.
Esperion Therapeutics Inc. is in the process of developing
Bempedoic acid as a small molecule which acts as an AMPK stimulant for the
treatment of hypercholesterolemia, dyslipidaemia, and hypertension. Betagenon
AB, Boehringer Ingelheim International GmbH, and Poxel SA are some other
companies having pipeline AMPK modulators for the management of several
diseases.
Request to Get the Sample Pages at:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials.
Pipeline analysis of drugs by phases includes product
description and development activities including information about clinical
results, designations, collaborations, licensing, grants, technology and
others.
Aurora Kinase Inhibitor Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 27, 2019and Other Developments, Aurora Kinase Inhibitor Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Collaborations, Designations, Patents
1 comment

Aurora
kinase are proteins that function as mitotic regulators and are
aberrantly expressed in cancerous cells. Aurora kinase inhibitors target these
aberrantly expressed regulators, provides genetic stability and prevents
tumorigenesis.
Several studies have demonstrated overexpression and
amplification of aurora kinase in various haematological and solid cancers.
Aurora kinase inhibitors when combined with other anti-cancer agents gives more
positive results in variety of cancers. Better evaluation of aurora kinase
inhibitors is required which raises the need of biomarkers, effective and novel
therapeutic agents. Also, aurora kinase inhibitors selectivity targets cancer
cells rather than healthy tissues, providing potential opportunities for more
effective drug development with reduced adverse effects.
Request to Get the Sample Pages at:
AstraZeneca PLC is in the process of developing AZD-2811 as
a small molecule which acts as an aurora B inhibitor for the treatment of solid
tumors. Some of the other companies having pipeline of aurora kinase inhibitors
include AbbVie Inc., Merck & Co., Inc., and CASI Pharmaceuticals Inc.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Hepatocyte Growth Factor (HGF) Inhibitor Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 24, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hepatocyte Growth Factor (HGF) Inhibitor Therapeutics - Pipeline Analysis 2019, Patents
1 comment

Hepatocyte
growth factor (HGF) is secreted by human pancreatic stellate cells and
present predominantly on cancer cells. Thus, HGF inhibitors has emerged as an
effective therapy in preventing local tumor growth. Also, studies have
demonstrated that HGF inhibitors are significantly more effective than
Gemcitabine in inhibiting tumor angiogenesis and metastasis.
Studies have demonstrated that the HGF inhibitor can be a
novel therapeutics that can be developed as a monotherapy or as combination
therapy with RAS-RAF-MEK, AKT-mTOR, and EGFR inhibitors for the treatment of
various human cancers. HGF inhibitors have shown potent antineoplastic effects
in several clinical trials for the treatment of head and neck squamous cell
carcinoma as HGF/c-Met pathway is directly involved in the development of a
proliferative, invasive, and metastatic cancer.
Request to Get the Sample Pages at:
AVEO Pharmaceuticals Inc. is in the process of developing
Ficlatuzumab as a potent HGF inhibitor antibody that binds to the HGF ligand
with high affinity and specificity to inhibit HGF/c-Met biological activities
for the treatment of squamous cell carcinoma of the head and neck and acute
myeloid leukemia. Galaxy Biotech LLC, Daiichi Sankyo Company Limited, and Taiho
Pharmaceutical Co. are some other companies having HGF inhibitor pipeline.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
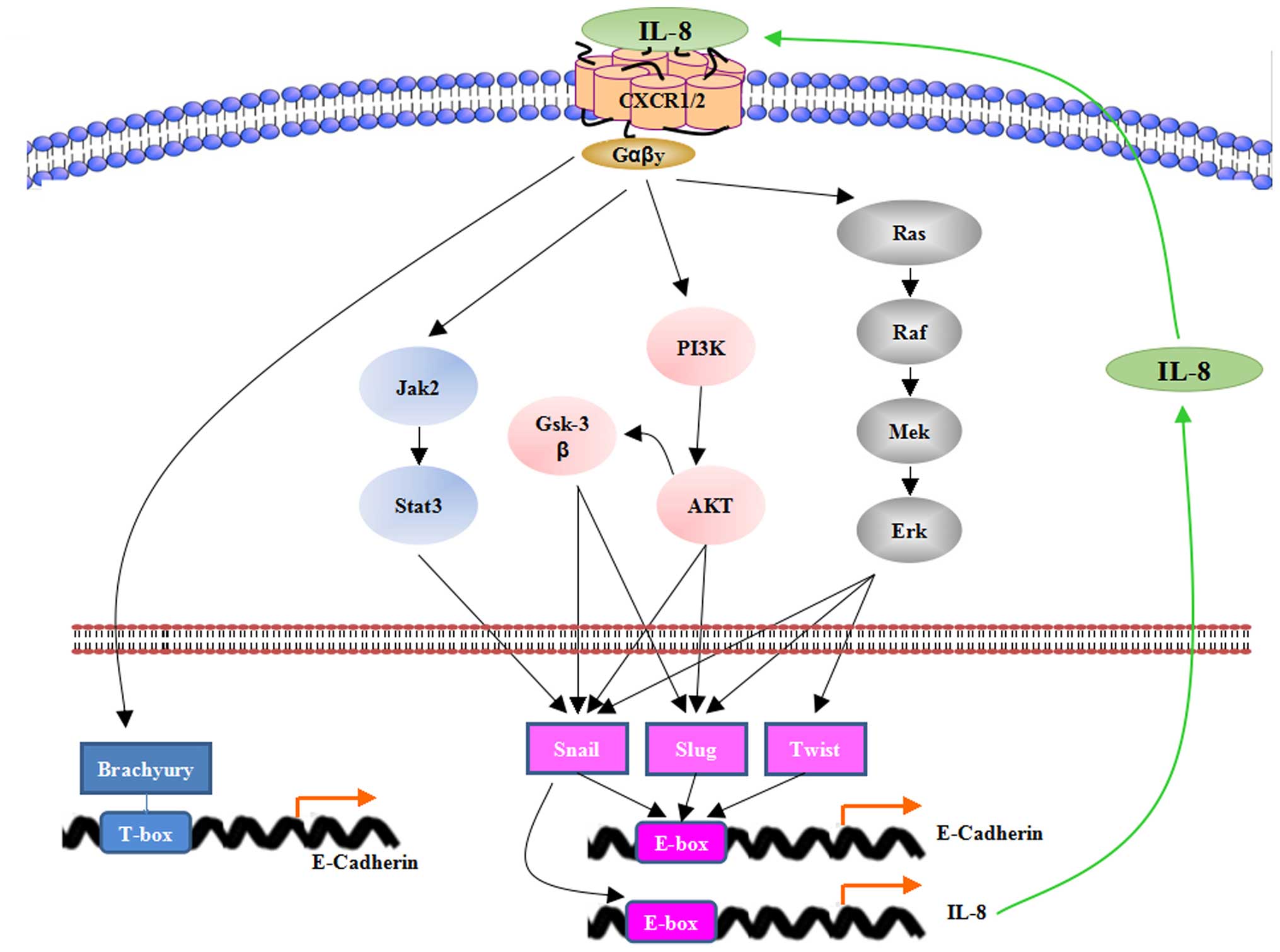
Interleukin 8 Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 24, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Interleukin 8 Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

Interleukin 8 is a
chemokine produced by various immune cells. They promote angiogenesis,
proliferation and development of cancerous cells. The other diseases which are
associated with interleukin 8 are depression, schizophrenia, bipolar disorder,
migraine, Alzheimer’s disease, colitis, rheumatoid arthritis, multiple
sclerosis, asthma, allergic rhinitis, psoriasis, eczema and others.
Interleukin 8 inhibitors therapies have shown improvements
as anti-CXCL8 signalling inhibitors for the treatment of various diseases,
including cancer and inflammatory diseases. Studies demonstrated that interleukin
8 can be indirectly targeted by mitogen activated protein kinase (MAPK)
inhibitors, phosphatidylinositol-3-kinase (PI3K)/AKT inhibitors, and NF-κB
inhibitors while direct targeting can be done by CXCL8 neutralizing antibodies,
and other drug therapies such as Reparixin. Potent effects of interleukin 8
inhibitors therapeutics as chemo-modulators and anti-angiogenics are expected
to treat various tumors.
Request to Get the Sample Pages at:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Mitogen-Activated Protein Kinase (MAPK) Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Mitogen-Activated Protein Kinase (MAPK) Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

Mitogen-activated
protein kinase (MAPK) are ubiquitous regulators of many cellular
functions including cell proliferation, cellular growth, inflammatory responses
to stress signals and cell differentiation. MAPK pathway, involves three main
kinases, Raf, MEK and ERK, has emerged as a novel target for the development of
new cancer therapies with reduced side-effects.
MAPK inhibitors are being developed as small molecules, for
the treatment of various types of cancer. They have also showed additional
benefits with high effectiveness at lower concentrations in comparison to the
ATP-competitive inhibitors, for the cancer therapy.
Request to Get the Sample Pages at:
Mereo BioPharma Group plc is in the process of developing
Acumapimod as an oral p38 MAPK inhibitor for the treatment of acute
exacerbations of chronic obstructive pulmonary disease (AECOPD). eFFECTOR
Therapeutics Inc., Genentech Inc., and Kura Oncology Inc. are some other
companies having pipeline of MAPK inhibitors.
Access Detailed Report Summary: https://www.pharmaproff.com/report/mapk-inhibitors-therapeutics
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
MDM2 Protein Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, MDM2 Protein Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

MDM2 protein
are powerful oncogene which is overexpressed in various cancers, including
breast cancer and sarcoma. There are many small molecule drug candidates that
are being developed as MDM2 protein inhibitors as monotherapy or combination
therapy for the treatment of various cancers.
Combination therapies are more
effective than monotherapy in certain cases. The therapeutic strategies aim at
blocking MDM2 expression, blocking the physical interaction between MDM2 and
p53, modulating the E3 ubiquitin ligase activity of MDM2 and targeting the
MDM2-p53 (protein–protein) complex, for the treatment of various indications.
Request to Get the Sample Pages at:
MDM2 protein therapies have shown positive clinical results
for the treatment of various cancers. Also, researches have demonstrated that
additional biomarkers are required to be identified to increase the chances of
clinical success as mutations in p53 can lead to resistance to MDM2 inhibitors.
Access Detailed Report Summary: https://www.pharmaproff.com/report/mdm2-protein-inhibitors
Daiichi Sankyo Company Limited is in the process of
developing DS-3032 as a proto-oncogene protein C MDM2 inhibitor for the
treatment of leukemia, and solid cancers. Some of the other companies having
pipeline of MDM2 protein inhibitors include Aileron Therapeutics Inc., Amgen
Inc., and F. Hoffmann-La Roche Ltd.
Interleukin Receptor Modulators Therapeutics - Pipeline Analysis 2019, Clinical Trials & Results, Patents
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Interleukin Receptor Modulators Therapeutics - Pipeline Analysis 2019, Patents
No comments

Interleukin
receptors are a group of cytokine receptors that are expressed by
leukocytes. Interleukin receptors play an important role in the functioning of
the immune system. However, with complete knowledge of their role in
pathogenesis of different diseases from allergic reactions to autoimmune
disorders and even cancer makes these interleukin receptors an attractive
target among different available treatment modalities.
Immune-modulatory role of interleukins as well as their
direct and indirect contact with apoptosis and other cancer developments,
angiogenesis and progression pathways makes them attractive targets for cancer
treatment. GlaxoSmithKline plc is in the process of developing GSK2618960 as an
interleukin 7 modulator for the treatment of Sjogren's syndrome. Some of the
other companies having pipeline of interleukin receptor modulators include
Can-Fite BioPharma Ltd., and Pfizer Inc.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licencing, grants, technology and others.
Endothelin Antagonists Therapeutics - Pipeline Analysis , Patents, Designations, Collaborations, and Other Developments
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Endothelin Antagonists Therapeutics - Pipeline Analysis 2019, Patents
No comments

Endothelin antagonists are drug candidates that acts against
endothelin receptors and generate pharmacological actions such as blocking the
vasoconstriction and mediate vasodilatation. Endothelin-1 is a peptide which is
comprised of 21 amino acids and formed by vascular endothelium. The biological
molecule is a potent vasoconstrictor that binds to endothelin receptors A and
B. This further leads to the activation of IP3 DA pathway that causes efflux of
calcium ions from the endoplasmic reticulum and produce vasoconstriction in the
smooth muscles.
AbbVie Inc. is in the process of developing ABT-627 as an
endothelin receptor antagonist for the treatment of diabetic nephropathy in
type 2 diabetes. Idorsia Pharmaceuticals Ltd. is developing ACT-132577 as an
endothelin receptor antagonist for the treatment of resistant hypertension.
Moreover, Retrophin Inc. is also developing sparsentan as an endothelin
receptor antagonist for the treatment of focal segmental glomerulosclerosis.
Ligand Pharmaceuticals Inc., and ENB Therapeutics LLC are some other companies
having pipeline of endothelin receptor antagonist.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with the detailed analysis of pipeline and clinical trials.
Pipeline analysis of drugs by phases includes product description and
development activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Epidermal Growth Factor Receptor (EGFR) Antagonists Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Epidermal Growth Factor Receptor (EGFR) Antagonists Therapeutics - Pipeline Analysis, Patents
No comments

Epidermal growth factor receptors (EGFR) are a group of
receptor tyrosine kinase (RTK) that plays a major role in cell proliferation,
survival and differentiation. In most of the cancer, EGFR are amplified and
overexpressed, due to dysregulation, resulting in cancer development.
EGFR inhibitors therapies have shown improvement by
inhibiting receptor signalling and enhancing the effects of radiation therapy
and conventional chemotherapy with their anti-tumor activity. Cetuximab,
Panitumumab, Erlotinib and Afatinib are some of the major marketed drugs as
anti-EGFR therapies for the treatment of colorectal cancer, head and neck
cancer and non-small cell lung cancer.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the pipeline
activities covering all drug candidates under various stages of development,
with detailed analysis of pipeline and clinical trials. Pipeline analysis of
drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licencing, grants, technology and others.
Galectin Inhibitor Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations, and Other Developments
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Galectin Inhibitor Therapeutics - Pipeline Analysis, Patents
No comments

Galectins belong to the family of animal lectins and perform
function by interacting with cytoplasmic and nuclear proteins, cell-surface and
extracellular matrix glycoproteins and glycolipids to modulate signalling
pathways. Studies have shown the crucial roles of galectin in cancer as they contribute
to tumour cell survival, tumour metastasis, angiogenesis and neoplastic
transformation.

Many studies have demonstrated role of galectins in the
pathogenesis of various diseases including malignant, fibrotic and inflammatory
diseases; hence, emerges as an attractive target for the therapeutic of various
diseases. Galectin-3 inhibitors has shown positive clinical results for the
treatment of acute lymphoblastic leukemia. Thus, providing several
opportunities to the companies for the development of galectin-based therapies
for the treatment of multiple human diseases.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Read More..