Adrenocortical Carcinoma Therapeutics - Pipeline Analysis, Collaborations, and Other Developments
Pramod KmrOctober 02, 2019Adrenocortical Carcinoma Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Developments, Diagnostics, Drugs Policy and Regulatory Landscape, Patents, Patient, Therapeutics
2 comments

Adrenocortical
carcinoma, also known as adrenal cortex carcinoma, is a rare form of
cancer that occurs at the cortex layer of the adrenal gland. There are two
adrenal glands present in the human body: one at the top of each kidney. Each
adrenal gland is comprised of two distinct structures: the outer part of the adrenal
glands (adrenal cortex) and the inner region (adrenal medulla).
Some of the genetic condition that can cause adrenocortical
carcinoma are Li-Fraumeni syndrome, Carney complex, and Beckwith-Wiedemann
syndrome. Some of the most common symptoms of this medical condition includes
abdominal pain, lump in the abdomen, and feeling of fullness.
Request to Get the Sample Pages at:
The disease can
be diagnosed by imaging technique such as computed tomography (CT) scan,
magnetic resonance imaging (MRI), biopsy, blood tests, and urine tests. Millendo
Therapeutics Inc. is in the process of developing ATR-101 as an acetyl CoA
C-acetyltransferase inhibitor for the treatment of adrenocortical carcinoma.
Merck & Co., Inc. is also in the process of developing pembrolizumab as a
CD274 antigen inhibitor for the treatment of adrenocortical carcinoma. Some of
the other companies and universities having the pipeline drug for
adrenocortical carcinoma includes the Columbia University, Medunik Canada Inc.,
HRA Pharma, and others.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with the detailed analysis of pipeline and clinical trials.
Pipeline analysis of drugs by phases includes product
description and development activities including information about clinical
results, designations, collaborations, licensing, grants, technology, and
others.
Cathepsin K Inhibitors Therapeutics - Pipeline Analysis 2019 Clinical Trials & Results
Pramod KmrOctober 02, 2019and Other Developments, Cathepsin K Inhibitors Therapeutics - Pipeline Analysis 2019, Clinical Trials & Results, Collaborations, Designations, Patents
1 comment

Cathepsin K is a protease enzyme which is coded by CTSK gene. The function associated with the gene involves bone remodelling and resorption. Cathepsin K is a member of peptidase C1 protein family and expressed mainly in osteoclasts. The protein has high specificity for kinins and has the capability to catabolize collagen, elastin, and gelatin. Cathepsin K is found to be over-expressed in cancer such as breast cancer and glioblastoma. The mutation in CTSK gene can cause pycnodysostosis. Due to over expression of cathepsin K in osteoclasts, bone deformation occurs which leads to osteoporosis.
Company like Medivir AB is in the process of developing MIV-711 as a cathepsin K inhibitor for the treatment of osteoarthritis. Amura Holdings Ltd., and Merck & Co. Inc. are some other companies having pipeline of cathepsin K inhibitors.
Request to Get the Sample Pages at:
The report provides a comprehensive understanding of the pipeline activities covering all drug candidates under various stages of development, with the detailed analysis of pipeline and clinical trials. Pipeline analysis of drugs by phases includes product description and development activities including information about clinical results, designations, collaborations, licensing, grants, technology, and others.
Adenosine 5-Monophosphate Activated Protein Kinase (AMPK) Modulators Therapeutics - Pipeline Analysis 2018
Pramod KmrSeptember 27, 2019Adenosine 5-Monophosphate Activated Protein Kinase (AMPK) Modulators Therapeutics - Pipeline Analysis 2018, and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents
1 comment

Adenosine
5-monophosphate activated protein kinase (AMPK) plays an important role
in regulation of homeostasis. Dysregulation of AMPK can result in many diseases
which includes obesity and type 2 diabetes. Several studies have also suggested
that AMPK are also associated with the development of neurological diseases and
cancer.
Esperion Therapeutics Inc. is in the process of developing
Bempedoic acid as a small molecule which acts as an AMPK stimulant for the
treatment of hypercholesterolemia, dyslipidaemia, and hypertension. Betagenon
AB, Boehringer Ingelheim International GmbH, and Poxel SA are some other
companies having pipeline AMPK modulators for the management of several
diseases.
Request to Get the Sample Pages at:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials.
Pipeline analysis of drugs by phases includes product
description and development activities including information about clinical
results, designations, collaborations, licensing, grants, technology and
others.
Aurora Kinase Inhibitor Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 27, 2019and Other Developments, Aurora Kinase Inhibitor Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Collaborations, Designations, Patents
1 comment

Aurora
kinase are proteins that function as mitotic regulators and are
aberrantly expressed in cancerous cells. Aurora kinase inhibitors target these
aberrantly expressed regulators, provides genetic stability and prevents
tumorigenesis.
Several studies have demonstrated overexpression and
amplification of aurora kinase in various haematological and solid cancers.
Aurora kinase inhibitors when combined with other anti-cancer agents gives more
positive results in variety of cancers. Better evaluation of aurora kinase
inhibitors is required which raises the need of biomarkers, effective and novel
therapeutic agents. Also, aurora kinase inhibitors selectivity targets cancer
cells rather than healthy tissues, providing potential opportunities for more
effective drug development with reduced adverse effects.
Request to Get the Sample Pages at:
AstraZeneca PLC is in the process of developing AZD-2811 as
a small molecule which acts as an aurora B inhibitor for the treatment of solid
tumors. Some of the other companies having pipeline of aurora kinase inhibitors
include AbbVie Inc., Merck & Co., Inc., and CASI Pharmaceuticals Inc.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Hepatocyte Growth Factor (HGF) Inhibitor Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 24, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hepatocyte Growth Factor (HGF) Inhibitor Therapeutics - Pipeline Analysis 2019, Patents
1 comment

Hepatocyte
growth factor (HGF) is secreted by human pancreatic stellate cells and
present predominantly on cancer cells. Thus, HGF inhibitors has emerged as an
effective therapy in preventing local tumor growth. Also, studies have
demonstrated that HGF inhibitors are significantly more effective than
Gemcitabine in inhibiting tumor angiogenesis and metastasis.
Studies have demonstrated that the HGF inhibitor can be a
novel therapeutics that can be developed as a monotherapy or as combination
therapy with RAS-RAF-MEK, AKT-mTOR, and EGFR inhibitors for the treatment of
various human cancers. HGF inhibitors have shown potent antineoplastic effects
in several clinical trials for the treatment of head and neck squamous cell
carcinoma as HGF/c-Met pathway is directly involved in the development of a
proliferative, invasive, and metastatic cancer.
Request to Get the Sample Pages at:
AVEO Pharmaceuticals Inc. is in the process of developing
Ficlatuzumab as a potent HGF inhibitor antibody that binds to the HGF ligand
with high affinity and specificity to inhibit HGF/c-Met biological activities
for the treatment of squamous cell carcinoma of the head and neck and acute
myeloid leukemia. Galaxy Biotech LLC, Daiichi Sankyo Company Limited, and Taiho
Pharmaceutical Co. are some other companies having HGF inhibitor pipeline.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
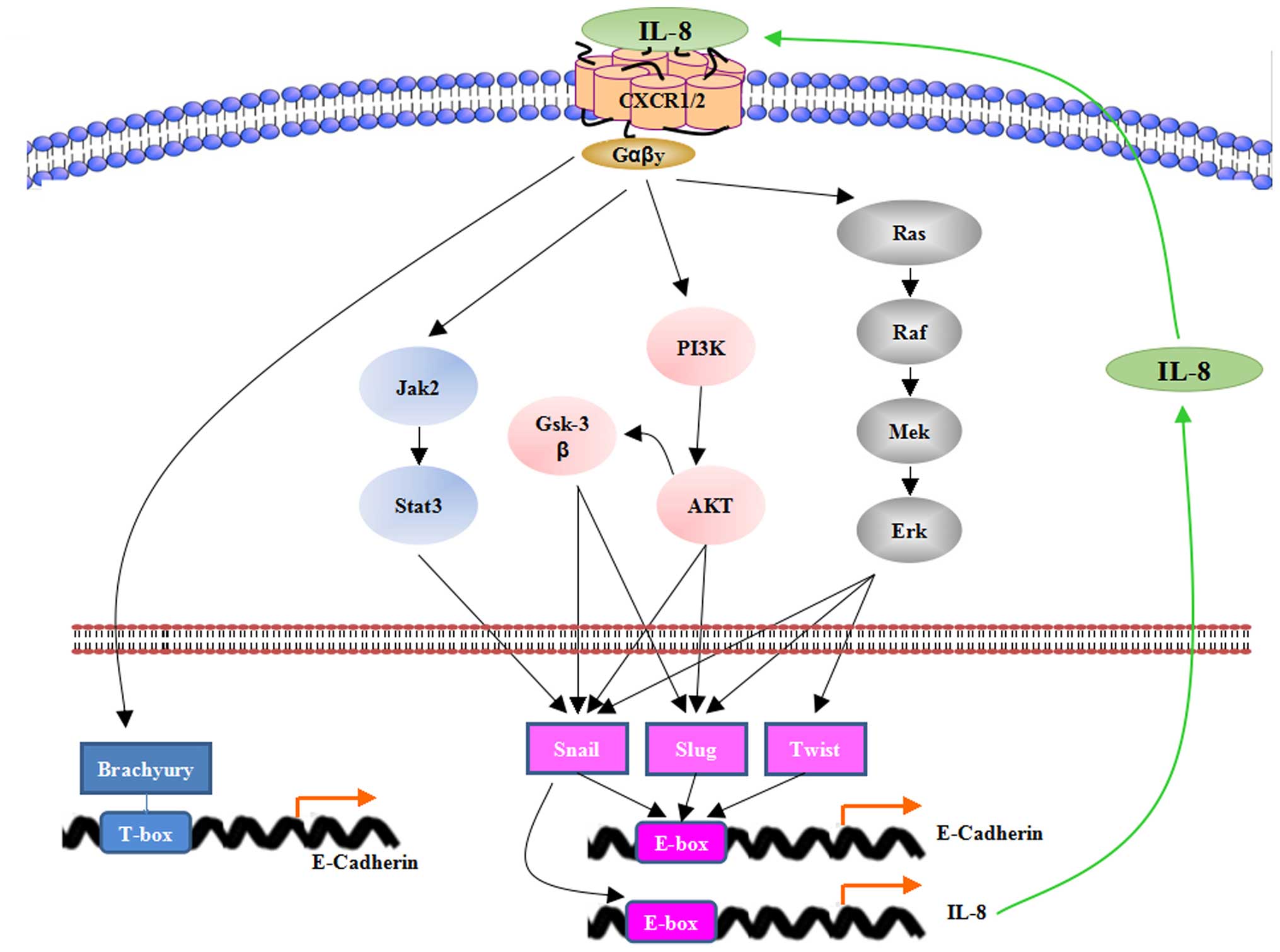
Interleukin 8 Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 24, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Interleukin 8 Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

Interleukin 8 is a
chemokine produced by various immune cells. They promote angiogenesis,
proliferation and development of cancerous cells. The other diseases which are
associated with interleukin 8 are depression, schizophrenia, bipolar disorder,
migraine, Alzheimer’s disease, colitis, rheumatoid arthritis, multiple
sclerosis, asthma, allergic rhinitis, psoriasis, eczema and others.
Interleukin 8 inhibitors therapies have shown improvements
as anti-CXCL8 signalling inhibitors for the treatment of various diseases,
including cancer and inflammatory diseases. Studies demonstrated that interleukin
8 can be indirectly targeted by mitogen activated protein kinase (MAPK)
inhibitors, phosphatidylinositol-3-kinase (PI3K)/AKT inhibitors, and NF-κB
inhibitors while direct targeting can be done by CXCL8 neutralizing antibodies,
and other drug therapies such as Reparixin. Potent effects of interleukin 8
inhibitors therapeutics as chemo-modulators and anti-angiogenics are expected
to treat various tumors.
Request to Get the Sample Pages at:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Mitogen-Activated Protein Kinase (MAPK) Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Mitogen-Activated Protein Kinase (MAPK) Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

Mitogen-activated
protein kinase (MAPK) are ubiquitous regulators of many cellular
functions including cell proliferation, cellular growth, inflammatory responses
to stress signals and cell differentiation. MAPK pathway, involves three main
kinases, Raf, MEK and ERK, has emerged as a novel target for the development of
new cancer therapies with reduced side-effects.
MAPK inhibitors are being developed as small molecules, for
the treatment of various types of cancer. They have also showed additional
benefits with high effectiveness at lower concentrations in comparison to the
ATP-competitive inhibitors, for the cancer therapy.
Request to Get the Sample Pages at:
Mereo BioPharma Group plc is in the process of developing
Acumapimod as an oral p38 MAPK inhibitor for the treatment of acute
exacerbations of chronic obstructive pulmonary disease (AECOPD). eFFECTOR
Therapeutics Inc., Genentech Inc., and Kura Oncology Inc. are some other
companies having pipeline of MAPK inhibitors.
Access Detailed Report Summary: https://www.pharmaproff.com/report/mapk-inhibitors-therapeutics
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
MDM2 Protein Inhibitors Therapeutics - Pipeline Analysis 2019
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, MDM2 Protein Inhibitors Therapeutics - Pipeline Analysis 2019, Patents
No comments

MDM2 protein
are powerful oncogene which is overexpressed in various cancers, including
breast cancer and sarcoma. There are many small molecule drug candidates that
are being developed as MDM2 protein inhibitors as monotherapy or combination
therapy for the treatment of various cancers.
Combination therapies are more
effective than monotherapy in certain cases. The therapeutic strategies aim at
blocking MDM2 expression, blocking the physical interaction between MDM2 and
p53, modulating the E3 ubiquitin ligase activity of MDM2 and targeting the
MDM2-p53 (protein–protein) complex, for the treatment of various indications.
Request to Get the Sample Pages at:
MDM2 protein therapies have shown positive clinical results
for the treatment of various cancers. Also, researches have demonstrated that
additional biomarkers are required to be identified to increase the chances of
clinical success as mutations in p53 can lead to resistance to MDM2 inhibitors.
Access Detailed Report Summary: https://www.pharmaproff.com/report/mdm2-protein-inhibitors
Daiichi Sankyo Company Limited is in the process of
developing DS-3032 as a proto-oncogene protein C MDM2 inhibitor for the
treatment of leukemia, and solid cancers. Some of the other companies having
pipeline of MDM2 protein inhibitors include Aileron Therapeutics Inc., Amgen
Inc., and F. Hoffmann-La Roche Ltd.
Interleukin Receptor Modulators Therapeutics - Pipeline Analysis 2019, Clinical Trials & Results, Patents
Pramod KmrSeptember 13, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Interleukin Receptor Modulators Therapeutics - Pipeline Analysis 2019, Patents
No comments

Interleukin
receptors are a group of cytokine receptors that are expressed by
leukocytes. Interleukin receptors play an important role in the functioning of
the immune system. However, with complete knowledge of their role in
pathogenesis of different diseases from allergic reactions to autoimmune
disorders and even cancer makes these interleukin receptors an attractive
target among different available treatment modalities.
Immune-modulatory role of interleukins as well as their
direct and indirect contact with apoptosis and other cancer developments,
angiogenesis and progression pathways makes them attractive targets for cancer
treatment. GlaxoSmithKline plc is in the process of developing GSK2618960 as an
interleukin 7 modulator for the treatment of Sjogren's syndrome. Some of the
other companies having pipeline of interleukin receptor modulators include
Can-Fite BioPharma Ltd., and Pfizer Inc.
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licencing, grants, technology and others.
Endothelin Antagonists Therapeutics - Pipeline Analysis , Patents, Designations, Collaborations, and Other Developments
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Endothelin Antagonists Therapeutics - Pipeline Analysis 2019, Patents
No comments

Endothelin antagonists are drug candidates that acts against
endothelin receptors and generate pharmacological actions such as blocking the
vasoconstriction and mediate vasodilatation. Endothelin-1 is a peptide which is
comprised of 21 amino acids and formed by vascular endothelium. The biological
molecule is a potent vasoconstrictor that binds to endothelin receptors A and
B. This further leads to the activation of IP3 DA pathway that causes efflux of
calcium ions from the endoplasmic reticulum and produce vasoconstriction in the
smooth muscles.
AbbVie Inc. is in the process of developing ABT-627 as an
endothelin receptor antagonist for the treatment of diabetic nephropathy in
type 2 diabetes. Idorsia Pharmaceuticals Ltd. is developing ACT-132577 as an
endothelin receptor antagonist for the treatment of resistant hypertension.
Moreover, Retrophin Inc. is also developing sparsentan as an endothelin
receptor antagonist for the treatment of focal segmental glomerulosclerosis.
Ligand Pharmaceuticals Inc., and ENB Therapeutics LLC are some other companies
having pipeline of endothelin receptor antagonist.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with the detailed analysis of pipeline and clinical trials.
Pipeline analysis of drugs by phases includes product description and
development activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Epidermal Growth Factor Receptor (EGFR) Antagonists Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Epidermal Growth Factor Receptor (EGFR) Antagonists Therapeutics - Pipeline Analysis, Patents
No comments

Epidermal growth factor receptors (EGFR) are a group of
receptor tyrosine kinase (RTK) that plays a major role in cell proliferation,
survival and differentiation. In most of the cancer, EGFR are amplified and
overexpressed, due to dysregulation, resulting in cancer development.
EGFR inhibitors therapies have shown improvement by
inhibiting receptor signalling and enhancing the effects of radiation therapy
and conventional chemotherapy with their anti-tumor activity. Cetuximab,
Panitumumab, Erlotinib and Afatinib are some of the major marketed drugs as
anti-EGFR therapies for the treatment of colorectal cancer, head and neck
cancer and non-small cell lung cancer.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the pipeline
activities covering all drug candidates under various stages of development,
with detailed analysis of pipeline and clinical trials. Pipeline analysis of
drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licencing, grants, technology and others.
Galectin Inhibitor Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations, and Other Developments
Pramod KmrSeptember 06, 2019and Other Developments, Clinical Trials & Results, Collaborations, Designations, Galectin Inhibitor Therapeutics - Pipeline Analysis, Patents
No comments

Galectins belong to the family of animal lectins and perform
function by interacting with cytoplasmic and nuclear proteins, cell-surface and
extracellular matrix glycoproteins and glycolipids to modulate signalling
pathways. Studies have shown the crucial roles of galectin in cancer as they contribute
to tumour cell survival, tumour metastasis, angiogenesis and neoplastic
transformation.

Many studies have demonstrated role of galectins in the
pathogenesis of various diseases including malignant, fibrotic and inflammatory
diseases; hence, emerges as an attractive target for the therapeutic of various
diseases. Galectin-3 inhibitors has shown positive clinical results for the
treatment of acute lymphoblastic leukemia. Thus, providing several
opportunities to the companies for the development of galectin-based therapies
for the treatment of multiple human diseases.
Download Free PDF Sample Copy:
The report provides a comprehensive understanding of the
pipeline activities covering all drug candidates under various stages of
development, with detailed analysis of pipeline and clinical trials. Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licencing, grants, technology and others.
Read More..
Prevalence of Skin Diseases Accelerating the Dermatology Drugs Sector Growth
Pramod KmrAugust 08, 2019dermatological drugs ppt, Dermatology, Dermatology Drugs Market new dermatology drugs 2019, dermatology drugs pdf, dermatology medications list, top dermatology drugs
No comments

The human skin functionality
decreases with age, which leads to problems such as reduced subcutaneous fat,
slow healing, raised sensitivity to ultraviolet (UV) radiation, and increased
susceptibility to skin infections.
Hence, the growing geriatric population results
in the prevalence of skin diseases, such as intertrigo, as
atrophy, pruritus, and
candidiasis infection.
All of these are being increasingly cured with pharmaceutical products, which is
resulting in the growth of the dermatology drugs sector.
Further, the number
of people aged 80 or above is expected to rise massively during the forecast
period. The data for the geriatric population along with the rising prevalence of
skin diseases in the elderly predicts a heavy need for dermatological products.
Thus, the growing aging population and increasing prevalence of skin diseases are
driving the dermatology drugs sector across the globe.
Surging Geriatric Population Supporting the Wet Age-Related Macular Degeneration Sector Growth
Pramod KmrMay 06, 2019analysis, Share, Size, Trends, Wet Age-Related Macular Degeneration Market, Wet Age-Related Macular Degeneration Market Deemand
8 comments

The growing prevalence of age-related macular degeneration
(AMD), lack of specific treatment availability, and increase in geriatric
population are driving the growth of the wet age-related macular degeneration
market. The market is projected to generate revenue worth $10.4 billion in
2024, witnessing a CAGR of 7.1% during the forecast period (2019–2024). AMD is a disease in which blurred vision or
blindness occurs due to damage to the retina macula. Wet AMD is a type of AMD
characterized by the growth of abnormal blood vessels under the retina and
macula.
On the basis of product, the wet
age-related macular degeneration market is categorized into Eylea,
Avastin, Lucentis, and others. Out of these, during the historical period
(2014–2018), the Eylea category witnessed the fastest growth, and it is
expected to keep growing during the forecast period at a CAGR of 10.0%. This is
mainly attributed to the surging demand for this medication due to its low cost
as well as high efficiency in the management of exudative or wet AMD.
Request to Get the Sample Pages at:
The risk of developing AMD is directly proportional to age.
With the number of people aged 60 years and above rising, the prevalence of AMD
is also increasing. According to the World Population Prospects data published
by the United Nations (UN), in 2017, there were about 962 million people in the
age group 60 years or over across the globe, and the number is predicted to
more than double by 2050, reaching 2.1 billion ultimately. Thus, it can be
concluded that the geriatric population is rising faster than younger age
groups.
Japan, Germany, Italy, and Portugal are among the nations
with the largest population of elderly in the world. As older people are more
prone to degenerative disorders, the increasing geriatric population in these
countries is anticipated to contribute to the rising demand for drugs for wet
AMD treatment, thus driving the wet age-related macular degeneration market
during the forecast period.
Therefore, the surging geriatric population across the world
is pushing the demand for drugs for treating wet AMD, resulting in the growth
of the market.
Uveitis Treatment Market Share Analysis of The Top Industry Players, Growth Opportunities and Country Level Segments
Pramod KmrApril 05, 2019Uveitis Treatment Market Demand, Uveitis Treatment Market Growth, Uveitis Treatment Market Outlook, Uveitis Treatment Market Share, Uveitis Treatment Market Size, Uveitis Treatment Market Trends
6 comments

Globally,
North America is the largest, while Asia-Pacific is the fastest growing uveitis
treatment market. The uveitis treatment market is witnessing significant
growth, due to increasing prevalence of uveitis and related complications. The
global uveitis treatment market currently has many technologically innovative
products in the pipeline, which are expected to improve efficiency and safety
profiles in the clinical trial stage of development, thereby reducing the
progression of disease considerably.
Anterior
uveitis represents the largest growing segment of global uveitis treatment
market, as per the type of uveitis. Anterior uveitis refers to redness in the
middle layer of an eye, which contains ciliary body and iris. Anterior uveitis
might be chronic or acute in nature. Anterior uveitis is caused primarily due
to fungal, bacterial or viral infections and auto-immune disorders. The
treatment for uveitis is primarily based on steroids and immunosuppressants.
The global uveitis treatment market is currently dominated by uveitis treatment
through corticosteroid (steroid medication) as well as off-label
immuno-suppressive and biologic drugs.
Request to Get the
Sample Pages at:
The global
uveitis treatment market is witnessing substantial growth due to increased
aging population, growing prevalence of uveitis and related complications.
Moreover, introduction of innovative biologics, fast-track approval of pipeline
drugs, increasing research and development investments in drug discovery and
development, rising government initiatives to minimize eye disorders are also
driving the growth of the global uveitis treatment market. Introduction of new
routes for drug administration that improves the efficiency of the drug is
another significant driver for uveitis treatment market. With the advancement
of molecular biology, some intravitreal implants and biologic agents are
increasingly used for the treatment of uveitis. By the development of molecular
biology, more therapeutic agents and approaches have been applied to control
ocular inflammation caused by uveitis, which are expected to have a positive
influence on the uveitis treatment market. The advancements anticipated from
the uveitis treatment options currently in the pipeline are sparking out
serious market growth.
Make Enquiry Before
Buying the Report: https://www.psmarketresearch.com/send-enquiry?enquiry-url=uveitis-treatment-market
However,
the adverse effects related to uveitis treatment medication such as watery
eyes, irritation and others are the factors which are expected to hinder growth
of the global uveitis treatment market. Poor primary healthcare infrastructure,
lack of awareness about eye disorders, and lack of health insurance in
developing countries are also obstructing the uveitis treatment market.
Some of the
major competitors in the global uveitis treatment market are XOMA, pSivida Corp., Novartis,
Allergan India Private Limited, Enzo Biochem Inc., AbbVie Inc., Bausch &
Lomb Incorporated, Ophthalix Inc., Santen Pharmaceutical Co., Ltd., Topivert
Ltd, HANALL and Regeneron Pharmaceuticals, Inc.
Protein A Resin Market to Witness the Highest Growth Globally in Coming Years
Pramod KmrApril 03, 2019Protein A Resin Market Demand, Protein A Resin Market Growth, Protein A Resin Market Outlook, Protein A Resin Market Share, Protein A Resin Market Size, Protein A Resin Market Trends
3 comments

The global
protein A resin market is expected to witness considerable growth during the
forecast period due to the increasing research and development activities and
increasing prevalence of chronic diseases. High traction for monoclonal
antibodies in biopharmaceutical industry is supporting the growth of the global
market. The increasing demand for monoclonal antibodies has fueled the need for
high capacity commercial scale purification of monoclonal antibodies.
Protein A
resins are designed for the simple and rapid purification of antibodies.
Therefore, an increase in demand for monoclonal antibodies implies an increase
in demand for protein A resins. The market for natural protein A resin was the
larger than recombinant protein A resin market in 2015; however, recombinant
protein A resin market is expected to witness the higher growth during the
forecast period.
Request to Get the
Sample Pages at:
Protein A,
a 56 kDa surface protein, is found on the cell wall of Staphylococcus aureus
bacterium. The protein has immunoglobulin-binding domains which bind to
proteins, mostly immunoglobulin G (IgG). Protein A binds to the Fc region or
Fab region of immunoglobulins, thus, increasingly being used for immune
precipitation or antibody purification applications. Protein A ligand is used
for the affinity chromatography step in commercial-scale production of
monoclonal antibodies. The key factors driving the use of protein A resin for
antibody purification are high antibody selectivity, increased antibody yield
and excellent antibody purity, and ease of operation in large as well as small
scale antibody purification.
Make Enquiry Before
Buying the Report: https://www.psmarketresearch.com/send-enquiry?enquiry-url=protein-a-resin-market
Geographically,
North America accounted for the largest share in the global protein A resin
market in 2015, owing to the large number of clinical trials for the monoclonal
antibody based therapeutics and increasing traction for biosimilars with the
recent U.S. Food and Drugs Administration in the region. The U.S. contributed
largest revenue to the North American as well as global protein A resin market.
The country is expected to remain the largest market for protein A resin
globally during the forecast period.
The protein
A resin market is expected to witness the highest growth in Asia-Pacific during
the forecast period. The highest growth in the region is attributed to the
increasing compliance for biosimilars and shifting global interest for
expanding biopharmaceutical manufacturing capacities in the emerging economies
of the region.
The key
players operating in the global protein A resin market are General Electric Company, Thermo Fisher Scientific
Inc., Merck KGaA, Tosoh Corporation, Repligen Corporation, Novasep, Expedeon
Ltd., and GenScript.
Pompe Disease Market - Epidemiology Analysis, Therapy, Growth and Demand Forecast to 2024
Pramod KmrMarch 29, 2019Pompe Disease Market Demand, Pompe Disease Market Growth, Pompe Disease Market Outlook, Pompe Disease Market Share, Pompe Disease Market Size
16 comments

The pompe
disease market is growing at a significant rate mainly due to special
provisions for orphan drugs. Regulatory assistance in emerging nations is one
of the major trends seen in the pompe disease market.
Pompe
disease is a type of lysergic acid diethylamide (LSD). LSD refers to a group of
diseases arising from the increase of specific substrates and is inherited
autosomal recessive disorders. Lysergic acid diethylamide is progressive and
having uneven age of onset and clinical symptoms. Pompe disease is a hereditary
and repeatedly incurable disorder caused by lack of acid alpha glucosidase, an
enzyme desired to split down glycogen in particular structures of our body.
Patients
having pompe disease have little amount of or no acid alpha glucosidase enzyme
activity, so break down of glycogen is not possible. The additional glycogen
builds up in the patient and is stored in skeletal muscle, heart and other
tissues, which causes the progressive indications of pompe disease. Increase of
glycogen in certain organs and tissues restraints normal functions of patient’s
organs. Pompe disease is also known by other names such as glycogen storage
disease type II, acid maltase deficiency, lysosomal alpha-glucosidase
deficiency, acid alpha-glucosidase deficiency and glycogenosis type II.
Request to Get the Sample Pages at:
Pompe disease is categorized into two broad categories as adult-onset pompe disease
and infantile onset pompe disease. Both the parents pass on one strangely
altered copy of the gene to their child. A parent with an altered copy of the
gene is known as a carrier and the disease can affect both men and women
irrespective of the gender. The disease is acquired by the individuals at the
time of birth only, but the onset of indications differs significantly. The
researchers have identified more than 300 genetic mutations that can cause
pompe disease. Enzyme replacement therapy and diet therapy are useful in
dropping the clinical demonstrations of the disease.
Make Enquiry Before
Buying the Report: https://www.psmarketresearch.com/send-enquiry?enquiry-url=pompe-disease-market
Government
regulations, such as orphan drug exclusivity and limited patient population are
the factors restraining the growth of the pompe disease market.
Some of the
competitors in the market are Genzyme Corporation, EpiVax, Inc., Amicus
Therapeutics, Audentes Therapeutics, BioMarin Pharmaceutical Inc., Valerion
Therapeutics, LLC, Oxyrane, and Sangamo BioSciences Inc.
Pancreatic Cancer Therapeutics Market - Epidemiology Analysis, Therapy, Development, Growth and Demand Forecast to 2024
Pramod KmrMarch 27, 2019Pancreatic Cancer Therapeutics Market Demand, Pancreatic Cancer Therapeutics Market Growth, Pancreatic Cancer Therapeutics Market Share, Pancreatic Cancer Therapeutics Market Size
3 comments

The global pancreatic cancer therapeutics market holds numerous
opportunities for the key players, as pancreatic cancer is a highly challenging
disease among all types of cancer, which has no specific treatment available
for it, as of now. Also, growing incidence of cancer and technological
advancements are supporting the growth of the global pancreatic cancer
therapeutics market growth.
Cancer
instigates uncountable growth of cells due to some abnormalities in the body.
Some of the common symptoms of pancreatic cancer include weight loss, diarrhea,
jaundice and abdominal pain. Cancer hampers the pancreas’ normal functioning,
with smoking being the major risk factor for pancreatic cancer. Diagnosis of
this type of cancer is done by patient history check and complete physical
examination, followed by urine, stool and blood tests. Also, personal or family
history of pancreatitis and BRCA2 mutation inclines a person to be affected by
pancreatic cancer.
Request to Get the
Sample Pages at:
In
developing countries, improvement in patient-centered care is being encouraged,
as governments are investing huge amount of funds. Increasing elderly
population base, high prevalence of pancreatic cancer and increase in foreign
investments may be observed, over the forecast period, in the developing
nations such as Asia-Pacific and Middle East Asia.
Make Enquiry Before
Buying the Report: https://www.psmarketresearch.com/send-enquiry?enquiry-url=pancreatic-cancer-therapeutics-market
The
investment in both segments of pancreatic cancers is significant. However, the
growing prevalence and growing reach of novel techniques in the developing
regions are the aspects likely to drive the growth of the exocrine category,
throughout the forecast period.
Presently,
the global pancreatic cancer therapeutics industry is merged in
nature, with only a few top companies seizing the major share of the market.
Some of the top companies operating in this market are Celgene Corporation; Eli
Lilly and Company; Amgen, Inc.; F. Hoffmann-La Roche AG; PharmaCyte Biotech,
Inc.; Novartis AG; Teva Pharmaceutical Industries Ltd.; Clovis Oncology;
Pfizer, Inc.; and Merck & Co., Inc.
Several
companies are concentrating on the introduction of technologically advanced
products, in order to obtain a significant share in the fast-growing medical
devices area. Also, collaborations, mergers and partnerships with well-established
players are the key approaches followed by top players to increase their market
share, during the forecast period.
Integrated CDSS Segment Expected to Realize Fastest Growth in the Global CDSS Market, During Forecast Period
Vijay KumarMarch 26, 2019CDSS Industry, CDSS Market, CDSS Market Trends, Clinical Decision Support System Market, Global CDSS Market
1 comment

The clinical
decision support system market size is expected to cross $1.5 billion by
2022, growing at a CAGR of 21.5% between 2016-2022. The CDSS market is driven
by five major factors, including growing
demand for advanced healthcare information systems, growing investments by HIT
players, growing need for remote patient monitoring services, and increasing
support from government organizations.
The massive unexplored CDSS across the globe has created
abundant opportunities for the major players to exploit the potential of the
CDSS market. Although, there has been significant development in the developed
economies of European Union (EU) and the U.S., the clinical decision support
system market is still at its nascent form, especially in the developing
countries of Asia, Latin America, and Africa. A high growth in numbers of end
user including hospitals, and pharmacy has been witnessed in the developing
countries of these regions, which provides immense growth opportunities for the
CDSS market.
The Asia-Pacific
market for CDSS is expected to grow at a CAGR of 23.2% between 2016-2022. The clinical
decision support system market in
Asia-Pacific is growing, due to growing geriatric population and upsurge in the occurrence rate of different chronic diseases. In
addition, the improving healthcare infrastructure, and favorable government
support, such as investments and grants has fueled the demand of HIT systems
including CDSS in the region. China is expected to grow at a CAGR of 27% during
the forecast period and reach $114.3 million by 2022, in the Asia-Pacific CDSS
market.
Explore
Report at@ https://www.psmarketresearch.com/market-analysis/clinical-decision-support-system-market
Some of the key companies operating in the CDSS market
include Cerner Corporation, RELX Group PLC, Epic Systems Corporation, General
Electric Company, McKesson Corporation, Zynx Health Incorporated, International
Business Machine Corporation, Siemens AG, Allscripts Healthcare Solutions
Inc., Meditech and Wolters Kluwer.
GLOBAL CDSS MARKET SEGMENTATION
By Product
- Integrated
CDSS Solutions
- Standalone
CDSS Solutions
By Application
- Drug
Dosing Support
- Clinical
Guidelines
- Clinical
Reminders
- Drug-Drug
Interactions
- Drug
Allergy Alerts
- Others
By Model
- Knowledge-based
CDSS Solutions
- Non-Knowledge-Based
CDSS Solutions
By Mode of Delivery
- On-Premises
- Web-Based
- Cloud-Based
By Component
- Software
- Hardware
- Services
By Healthcare Provider Entity Capacity
- Less
than 100 Beds
- 100–199
Beds
- 200–299
Beds
- 300–399
Beds
- 400–499
Beds
- More
than 500 Beds
By Region
- North
America
- U.S.
- Canada
- Rest
of North America
- Western
Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Rest
of Western Europe
- Central
& Eastern Europe
- Russia
- Rest
of Central & Eastern Europe
- Asia-Pacific
- China
- Japan
- India
- Australia
- Rest
of Asia-Pacific
- Latin
America
- Brazil
- Rest
of Latin America
- Middle East and Africa
Source: P&S Intelligence
Pain Management Drugs Market Segmental Analysis by Therapeutics, Diagnostics, Patient, Drugs Policy and Regulatory Landscape
Pramod KmrMarch 07, 2019Pain Management Drugs Market, Pain Management Drugs Market Demand, Pain Management Drugs Market Share, Pain Management Drugs Market Size, Pain Management Drugs Market Trends, Research Report
3 comments

The growing
geriatric population and increasing demand of innovative and advanced pain
relaxing medications around the world are the predominant growth drivers for
the global pain
management drugs market. Additionally, the increasing number of
hospitalization cases; unmet requirements for neuropathic pain management
drugs; innovative and advanced applications of pain management therapies;
increasing prevalence of various chronic diseases, such as cancer, and
neurological problems; and increasing healthcare expenditure are also driving
the growth of the global market. The growing numbers of mergers and
acquisitions is a key trend observed in the market. Among the various
therapeutic indications, the post-operative pain relief segment accounted for
the largest share, and the low-back pain segment accounted for the second
largest share in the global market.
Pain
management drugs are mainly used to relieve discomfort associated with injury
and surgeries. Moreover, pain management medications are used in the management
of pain associated with neurological problems, migraine, cancer, orthopedic
problems, low- back pain, rheumatoid arthritis, and fibromyalgia.
Request to Get the
Sample Pages at:
North
America and Europe are the major markets, due to increasing prevalence of
chronic diseases, and growing awareness about various types of chronic pain
conditions in these regions. The U.S. followed by Canada, is the largest market
for pain management drugs in North America. Whereas, the U.K., Germany and
France are some of the major countries holding significant share in the
European pain management drugs market.
The Asian
market is growing with a significant rate, owing to huge pool of patients, and
increasing healthcare spending in the region. In addition, the initiatives
taken by various government associations to develop chronic pain rehabilitation
centers, and increasing prevalence of various chronic diseases are also
supporting the growth of the Asian pain management drugs market. The countries
such as India, Japan and China, are the major markets in the region.
Make Enquiry Before
Buying the Report: https://www.psmarketresearch.com/send-enquiry?enquiry-url=pain-management-drugs-market
Apart from
these regions, Latin America is another important market. This is due to
increasing investments by drug manufacturing companies and growing demand of
pain management medications in the region. Brazil holds the largest share in
the Latin American pain management drugs market, due to the increasing support
from government organizations for the development of chronic pain
rehabilitation center in the country.
Some of the
major players operating in the global pain management drugs market include
Purdue Pharma L.P., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company,
Forest Laboratories Inc., Endo International PLC, Johnson & Johnson, Pfizer
Inc., Valeant Pharmaceuticals International Inc., GlaxoSmithKline PLC, F.
Hoffmann-La Roche Ltd., and Baxter International Inc.
Pain
Management Drugs Market Segmentation
By Category
• Prescription-Based Drugs
• Over-The-Counter (OTC) Drugs
By
Therapeutic Indications
• Post-Operative Pain
• Low-Back Pain
• Rheumatoid Arthritis Pain
• Osteoarthritis Pain
• Cancer Pain
• Migraine
• Neuropathic Pain
• Fibromyalgia
• Others
By Brands
• Maxalt
• Zomig
• Qutenza
• Lidoderm
• Savella
• Imitrex
• Voltaren Gel
• Celebrex
• Cymbalta
• Others
By
Geography
• North America
o U.S.
o Canada
• Europe
o Germany
o France
o U.K.
o Spain
o Italy
• Asia
o Japan
o China
o India
• Latin America
o Brazil
• Rest of the World